2020. 3. 18. 22:10ㆍ카테고리 없음
Related Topics:Mole, Mass & Avogadro Constant. An amount of substance containing 6.02 × 10 23 particles is called a mole (often abbreviated to mol).
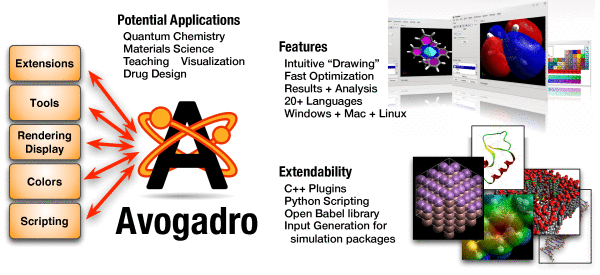
Using Avogadro Program For Kids
6.02 × 10 23 is called the Avogadro Constant or Avogadro's Number.The following diagram shows how to convert between Mass, Mole and Number of particles. Example:What is the molar mass of ethanol (C 2H 5OH)?Solution:Step 1: Look up the relative atomic masses of the atoms from the periodic table.Relative atomic mass (rounded to the nearest whole number):H = 1, C = 12, O = 16Step 2: Calculate the relative formula mass.Ethanol (C 2H 5OH) contains two carbon atoms, six hydrogen atoms and one oxygen atom.Relative formula mass = (2 × 12) + (6 × 1) + 16 = 46Step 3: Express the relative formula mass in grams per mole.The molar mass of ethanol is 46 g/mol Introduction to MolesA mole is like a dozen. It is a name for a specific number of things.
Avogadro Program Chemical Database
There are 12 things in a dozen, and 602 hexillion things in a mole.What are moles and why they are important?How to abbreviate the mole number (Avogadro's number) using scientific notation, and compare to see how giant this number is.